Medical Device Certification
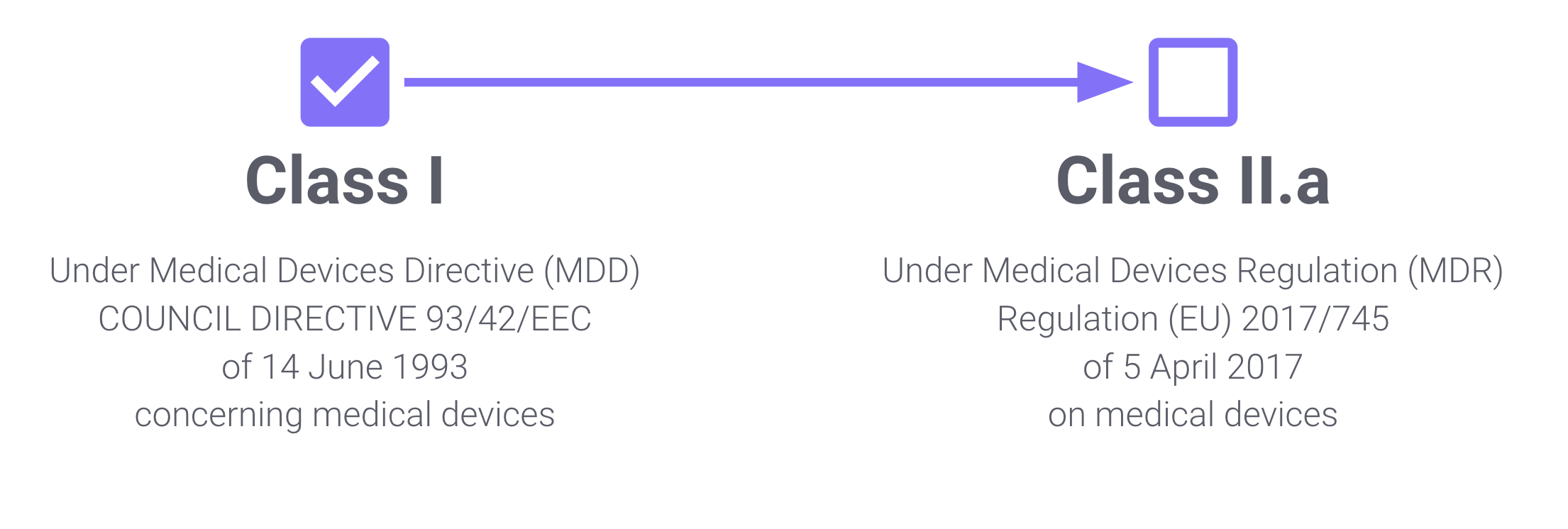
We got our first regulatory approval on 2020, while the MDD was in force. Under this regulation, our solution is a Class I medical device. However, under the current MDR, the solution is a Class II.a medical device.
note
Legally we have six years to adapt to the new MDR, but we are already in the transition with a notify body and we expect to have the Class II.a in 2024. For this, we are working with the consulting firm PS Consulting, from Madrid, and the notifying body is BSI.